PRE-CLINICAL STUDIES

Bioavailability
The effectiveness of LPT (Liposome Technology) ensures high bioavailability of cannabidiol, offering a significant advantage over other common delivery methods such as oral, sublingual, or inhalation. In a previously conducted clinical pilot study on osteoarthritic dogs, results demonstrated that LPT-CBD achieved exceptionally high drug bioavailability over the study period, calculated to approach 100% when compared to previously published pharmacokinetic data of CBD administrated intravenously to healthy dogs. In contrast, oral CBD administration was associated with a bioavailability range of only 6.5% to 20%.
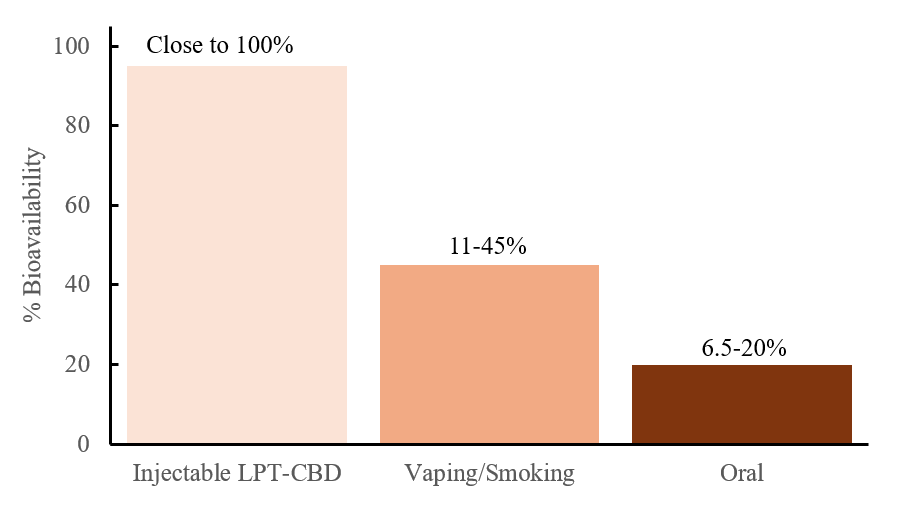
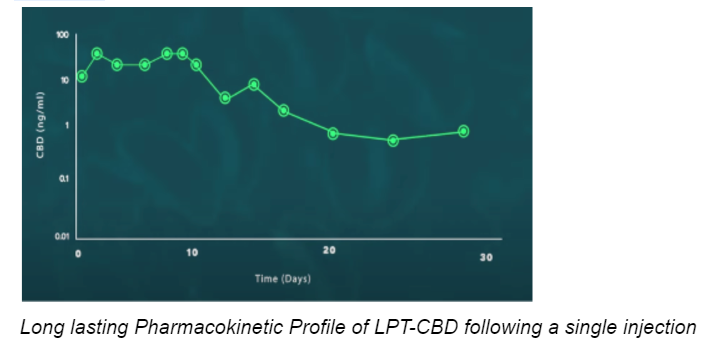
Use of CBD-loaded Liposome technology ensures the prolonged and controlled release of synthetic CBD into the bloodstream after a single injection. Our tests have shown that one LPT-CBD injection under the skin may be equivalent to administering oral CBD twice a day for 30 days.
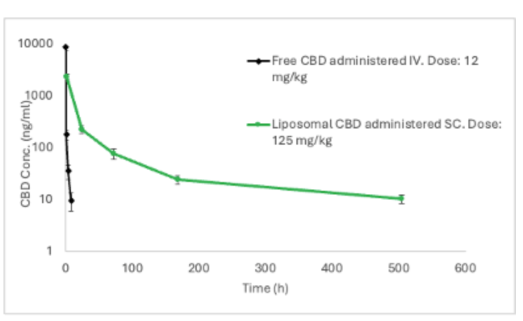
Pharmacokinetic Profile of Innocan subcutaneous injectable LPT-CBD compared to direct intravenous injection of Free CBD
Efficacy and Preliminary Safety Testing in Animal Models
Anti-inflammatory Study in Mice
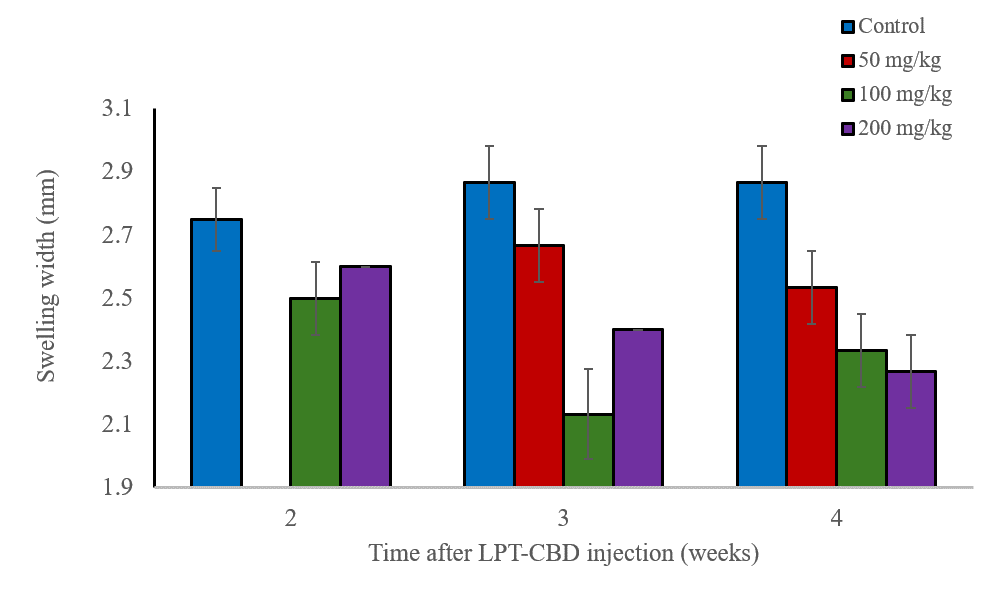
We evaluated the effect of LPT-CBD on an inflammatory response induced in mice. Inflammation, which may occur during mechanical injury of tissues, can be partially involved with the occurrence of pain. In this study, mice were subcutaneously injected with various concentrations of LPT-CBD. After 2, 3, and 4 weeks from LPT-CBD injection the mice were stimulated for a local inflammation in their paws. After 24 hours, their paw was evaluated for swelling compared to a group of naïve mice who served as control.
The results, shown in the figure below, indicate that injection of LPT-CBD at the doses tested and after 2, 3 and 4 weeks from administration, significantly reduced paw swelling. These results suggest that a single LPT-CBD injection provides a prolonged effect which can be maintained for at least 4 weeks.
Pharmacokinetic, Preliminary safety and Pain Relief Study in Clinical Dogs Suffering from Osteoarthritis
Pharmacokinetic results show that:
- A single subcutaneous administration of LPT-CBD provides long-term CBD plasma concentrations for up to 6 weeks.
- LPT-CBD provides slow drug release, as evidenced by a prolonged time to reach Cmax, indicating a sustained plasma profile.
- Pharmacokinetic calculations of the drug indicated an area under the curve (AUC) value normalized to dose of 1,393 ng*h/mL/mg/kg and 2,351 ng*h/ml/mg/kg (median value).
- These values correspond to 100% bioavailability when compared to AUC value normalized to dose reported in the literature for intravenous CBD administration in dogs.
Pain Evaluation indicated analgesic effect, lasting several weeks, as evidenced by:
- Significant improvement in dogs’ pain scoring.
- Significant improvement in dogs’ quality of life.
- Significant improvement in dogs’ activity.
Preliminary safety evaluation resulted in:
- Vital signs – No evidence for abnormal exceptions from baseline.
- Hematology – Most median hematology values were within reference ranges. Some parameters changed significantly from baseline, but changes were not clinically important, as values were kept within the reference range.
- Serum Biochemistry – Most median serum biochemistry values were within reference ranges. Some parameters changed significantly from baseline, but changes were not clinically important. Liver enzyme ALP level was not changed significantly during the study.
- Local Reaction – local swelling at the injection site was observed and resolved within 3-6 days without any treatment.
- Adverse events – No adverse events related to LPT-CBD were observed.
Study results can be found in the linked publications:
Pharmacokinetic and preliminary safety study in Gottingen Minipigs
The anatomical, physiological, and biochemical similarities of minipigs to humans make them a valuable translational model for pharmacokinetics, toxicity, and drug evaluation.
Pharmacokinetic results show that:
- A single subcutaneous administration of LPT-CBD at ascending doses of 5, 7.5, and 10 mg/kg results in sustained plasma concentrations of CBD and its primary human metabolite, 7-Carboxy-CBD (7-COOH-CBD), for up to 28 days.
LPT-CBD was well tolerated by the minipigs as evidenced by:
- Normal age-related weight gain and growth observed throughout the study period.
- No adverse effects observed throughout the study period.
- No local swelling was observed at the injection site.
- Histopathology evaluation of the injection sites revealed minimal to moderate reaction that was considered as non-adverse normal response to the injection.
- Most hematology and biochemistry values were within reference ranges. Values that showed deviations from the reference range were minor and of no clinical significance.
- Minipigs maintained a normal level of liver enzymes throughout the study.
Study results can be found in the linked publication:
Donkey Laminitis
As part of an observational study, we administered a liposomal-CBD injection to an amputee female donkey suffering from Laminitis in its left front limb. This innovative therapy provided immediate, noticeable pain relief and improved mobility.
Goat Scoliosis
In an observational study, we treated a 4-year-old male goat with a liposomal-CBD injection. The LPT-CBD therapy offered significant relief to the animal, which was born with neurological defects and scoliosis, resulting in hind-limb paralysis and fore-limb deformity.
Canine Polyarthritis
A compassionate care trial improved walking abilities and significantly decreased pain in participating canines, in an observational study. An 11-year-old spayed dachshund mix suffering from severe autoimmune-mediated polyarthritis, which caused almost complete inability to walk and severe pain, was included in the trial. Despite treatment with joint supplements (Glycoflex), steroids, and hydrotherapy, the dog remained very lame and could only walk a few steps. After administering Innocan’s liposomal CBD injection in addition to the other treatments, the dog showed remarkable improvement, walking longer distances and much faster than before.
Email us - [email protected]
ABOUT
Management
Contact Us
Le casino le plus payant en France
Testez le casino en ligne le plus payant France et profitez de jeux à forte rentabilité. Une expérience premium avec des retraits rapides et sûrs.

