REGULATORY PIPELINE

Government Regulation and FDA Regulatory Pathway
The Food and Drug Administration (FDA) oversees the rigorous process of approving new drugs and treatments in the United States, ensuring they meet stringent safety, efficacy, and quality standards before reaching the market. This regulatory pathway is vital for protecting public health and maintaining trust in the pharmaceutical and wellness industries. As we continue developing Liposome-based products for both human and veterinary use, we are committed to aligning with the FDA’s comprehensive requirements and strict process.
Our company is adopting a dual-track approach to FDA submissions, pursuing approval for human and veterinary applications. This strategy underscores our commitment to expanding the benefits of our LPT-based innovations to addressing chronic pain conditions, ensuring that both humans and animals can access safe and effective treatments.
Veterinary Application of Liposome-based Technology
Our LPT injection is designed as a veterinary drug aimed at treating companion animals experiencing chronic pain. The FDA’s Center for Veterinary Medicine (CVM) has granted a fee waiver and assigned an Investigational New Animal Drug (INAD) number for our LPT-CBD product.
This follows a series of pre-clinical trials conducted, including a study utilizing LPT-CBD on companion dogs with osteoarthritis which demonstrated significant pain relief. In connection with these results, we have conducted feasibility studies on pharmacokinetics and safety in healthy dogs.
Human Application of Liposome-based Technology
We have received positive feedback from the FDA following a pre-IND Type B meeting, with the agency acknowledged that LPT-CBD can be submitted under the 505(b)(2) pathway. This regulatory path can accelerate approval, leveraging existing data to facilitate a quicker route to commercialization.
Our comprehensive pre-IND package details extensive experiments demonstrating long-lasting pharmacokinetics and favorable tolerability of cannabidiol-loaded LPT (LPT-CBD) when used for an extended period (1-9 months) in small as well as big animals such as mouse, dogs and goats.
In parallel, we plan to conduct a full CMC development for LPT-CBD including locking drug specifications, scale-up and production under GMP to allow the execution of both toxicology and phase 1 clinical studies, finalizing the development of analytics methods and verifying/validating them for batch release, controlled LPT-CBD stability studies and final liposomal drug characterization as required by FDA’s liposomal drug development guidance. We intend to prepare and submit a CMC package for pre-IND meeting with the FDA.
Regulatory Pathway
A detailed graphic below outlines the process and current status of our regulatory pathway:
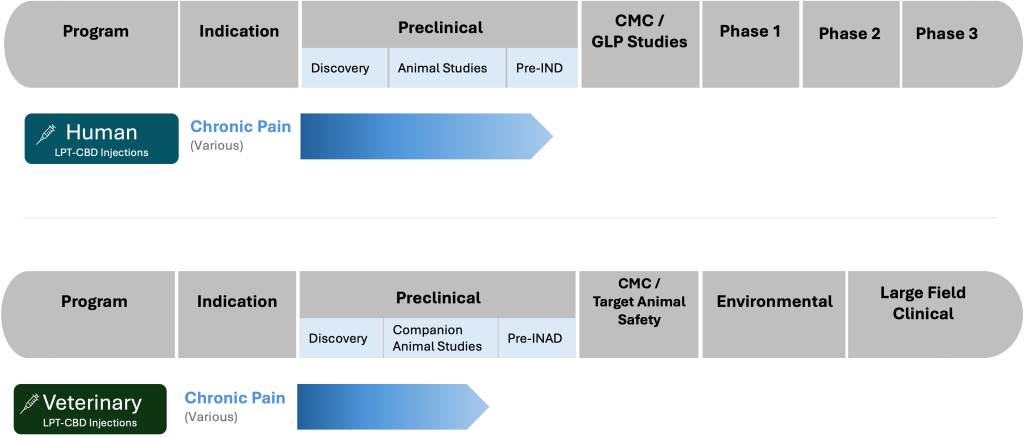
In addition to our pharmaceutical endeavors, we are equally dedicated to adhering to the regulatory requirements for wellness products. While the oversight for these products differs from pharmaceuticals, our commitment to quality, safety, and compliance remains unwavering. We rigorously test and validate the efficacy of our wellness offerings, ensuring they meet the high standards consumers expect and deserve.
By navigating these regulatory paths with diligence and precision, we are poised to make significant contributions to health and wellness, demonstrating our unwavering commitment to innovation, safety, and regulatory excellence.

